45 open-label meaning
dictionary.cambridge.org › dictionary › englishMILD | meaning, definition in Cambridge English Dictionary mild definition: 1. not violent, severe, or extreme: 2. Mild weather is not very cold or not as cold as usual: 3…. Learn more. HIV Risk and Prevention Estimates Overview. This section of the CDC HIV Web site provides public health professionals and others with the state of the science regarding HIV prevention including estimates from the current scientific literature for HIV risk behaviors, effective prevention strategies to reduce the risk of acquiring or transmitting HIV, and factors increasing HIV risk. ...
ComboBox.Label Property (DocumentFormat.OpenXml.Office2010.CustomUI ... label.Represents the following attribute in the schema: label

Open-label meaning
› dictionary › labelLabel Definition & Meaning - Merriam-Webster label: [verb] to affix a label to. to describe or designate with or as if with a label. What does open-label mean? - definitions Meaning of open-label. What does open-label mean? Information and translations of open-label in the most comprehensive dictionary definitions resource on the web. Login . Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1]
Open-label meaning. Open-label - definition of open-label by The Free Dictionary open-label Also found in: Medical, Wikipedia . o·pen-la·bel (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo. American Heritage® Dictionary of the English Language, Fifth Edition. › low-carb › ketoA Keto Diet for Beginners: The #1 Ketogenic Guide - Diet Doctor Sep 16, 2022 · A keto diet is a very low-carb, high-fat diet. You eat fewer carbs and replace it with fat, resulting in a state called ketosis. Get started on keto with delicious recipes, amazing meal plans, health advice, and inspiring videos to help you succeed. › dictionary › openOpen Definition & Meaning - Merriam-Webster open: [adjective] having no enclosing or confining barrier : accessible on all or nearly all sides. Open-label Definitions | What does open-label mean? | Best 2 ... Define open-label. Open-label as a adjective means Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatmen....
Cassava Sciences Reports Second Quarter Financial Results for Aug 03, 2022 · Open-label Study – Results of an Interim Analysis on the First 100 Patients Who Have Completed at Least 12 Months of Open-label Treatment with Simufilam Follow: Drug Appears Safe and Well Tolerated. CBD for Parkinson’s Disease Symptoms | APDA In a second trial, an open-label study of CBD was conducted on four patients with REM behavior sleep disorder. Symptoms decreased. A third trial was conducted on 21 patients with PD and was double blinded, meaning neither patient nor doctor knew who received treatment and who received a placebo. Bristol stool scale - Wikipedia The Bristol stool scale is a diagnostic medical tool designed to classify the form of human faeces into seven categories. It is used in both clinical and experimental fields.. It was developed at the Bristol Royal Infirmary as a clinical assessment tool in 1997, and is widely used as a research tool to evaluate the effectiveness of treatments for various diseases of the bowel, as well as a ... Relative risk - Wikipedia Statistical use and meaning. Relative risk is used in the statistical analysis of the data of ecological, cohort, medical and intervention studies, to estimate the strength of the association between exposures (treatments or risk factors) and outcomes. Mathematically, it is the incidence rate of the outcome in the exposed group, , divided by the rate of the unexposed group, .
Infections in patients with rheumatoid arthritis receiving tofacitinib ... Aug 02, 2022 · Objectives To characterise infections in patients with rheumatoid arthritis (RA) in ORAL Surveillance. Methods In this open-label, randomised controlled trial, patients with RA aged≥50 years with ≥1 additional cardiovascular risk factor received tofacitinib 5 or 10 mg two times per day or a tumour necrosis factor inhibitor (TNFi). Incidence rates (IRs; patients with … Medical Definition of Open-label - MedicineNet Open-label: A term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving. CONTINUE SCROLLING OR CLICK HERE SLIDESHOW Open-label Definition & Meaning - Merriam-Webster Definition of open-label : being or relating to a clinical trial in which the treatment given to each subject is not concealed from either the researchers or the subject an open-label multicenter study — compare double-blind, single-blind First Known Use of open-label 1979, in the meaning defined above Learn More About open-label myheart.net › articles › treatment-of-high-bloodTreatment of High Blood Pressure: When Medicines Don’t Work Mar 08, 2017 · This is an open-label, multi center, First-in-Man clinical trial that is conducted in the U.S. Patients with stage II resistant hypertension, that are treated with a minimum of 3 antihypertensive medications including a diuretic and who consent to participate in this study are assigned to treatment with a MobiusHD system (Vascular Dynamics ...
Mereo BioPharma Reports Clinical Update and Interim Biomarker … Sep 12, 2022 · Mereo’s lead oncology product candidate, etigilimab (anti-TIGIT), is currently in an open label Phase 1b/2 basket study evaluating anti-TIGIT in combination with an anti-PD-1 in a range of tumor types including three rare tumors and three gynecological carcinomas, cervical, ovarian, and endometrial carcinomas.
Open-label study | definition of open-label study by Medical dictionary open-label study a study in which there is no blinding of treatments. Farlex Partner Medical Dictionary © Farlex 2012 open-label study A clinical study in which the patients/subjects and investigators know which product each patient/subject is receiving, which is the opposite of a blinded study. Segen's Medical Dictionary. © 2012 Farlex, Inc.
What is an open label trial? | The BMJ Open label trials are sometimes referred to as "non-masked" or "unblinded." If the trial is a non-pharmacological study, such as a trial of devices, or psychological and physical treatments, it may be referred to simply as "open." After recruitment to the trial, the participants were allocated to treatment using block randomisation.
Understanding Clinical Trial Terminology: What is a Long-Term Extension ... An OLE study is a clinical trial that typically enrolls participants of a previous clinical trial and is designed to gather the long-term safety and tolerability data on a potential new medicine beyond the time period of the main study.
Open-label extension studies: do they provide meaningful ... - PubMed Open-label extension studies do have a legitimate but limited place in the clinical development of new medicines. The negative perceptions about these studies have arisen because of perversion of acceptable rationales for this type of study and a failure to recognise (or disclose) the limitations resulting from the inherent weaknesses in their ...
alsnewstoday.com › columns › clinical-trial-broughtHow a Clinical Trial Brought Meaning and Hope to Our ALS ... Sep 16, 2022 · But after five months of participation, Jeff exercised the option to continue in the study through open-label extension, taking the active drug itself. Jeff affectionately named the drug “Stink Drink” because it tasted terrible, but he loved being part of the trial.
FULL SPECTRUM CBD+THC GUMMIES - JustCBD Store Jun 17, 2022 · Our THC + CBD gummies are full-spectrum formulated, meaning they offer many other compounds alongside them. Commenting on hemp extracts, ... C. (2018). A prospective open‐label trial of a CBD/THC cannabis oil in dravet syndrome. Annals of Clinical and Translational Neurology, 5(9), 1077-1088.
Open-Label Trial | NIH - HIV.gov A type of clinical trial. In open-label trials, both the researchers and participants know which drug (or other intervention) is being given to participants.
Meaning of breakthrough in English - Cambridge breakthrough definition: 1. an important discovery or event that helps to improve a situation or provide an answer to a…. Learn more.
Understanding Clinical Trial Terminology: What is an Open Label ... Alternatively, sometimes, trials are conducted in an open-label fashion, meaning study participants and researchers both know which treatment the patient is receiving. Open-label trials can be used to compare treatments or gather additional information about the long-term effects in the intended patient population.
Open-label | definition of open-label by Medical dictionary Open-label | definition of open-label by Medical dictionary open-label Also found in: Dictionary, Wikipedia . open-label (ō′pən-lā′bəl) adj. Of or related to a clinical trial in which both the researcher and the participant know whether an administered treatment is an experimental drug or a placebo.
PDF On-Label vs. Off-Label Prescribing - IG Living label doesn't mean FDA disapproves of an off-label use. Rather, the agency just hasn't reviewed that use. While prescribing off-label can be challenging for physicians, they have the discretion to do so; however, they are not free to promote the off-label use.5 Drugs that are commonly prescribed off-label include antide-
Meaning of "Open Label Pilot study"..?? - SAS Open label is a term used to describe the situation when both the researcher and the participant in a research study know the treatment the participant is receiving. Open-label is the opposite of double-blind when neither the researcher nor the participant knows what treatment the participant is receiving.
Open label extension studies: research or marketing? - PMC Open label extension studies allow continued prescribing of unlicensed drugs after a randomised trial, but it is unclear whether patients or drug companies are benefiting the most Properly designed and conducted open label extension studies can provide rigorous information on long term safety and tolerability of potential new drugs.
PDF Blinding Sponsors for Open Label Studies: Challenges and Solutions - MWSUG In open label studies, in addition to treatment assignment data itself, many other data elements in the CRF data can reveal treatment assignment. Those data elements include (but not limited to): • A common variable that is collected for all treatment arms but takes different values for different treatment arms.
en.wikipedia.org › wiki › PlaceboPlacebo - Wikipedia In research trials. Knowingly giving a person a placebo when there is an effective treatment available is a bioethically complex issue. While placebo-controlled trials might provide information about the effectiveness of a treatment, it denies some patients what could be the best available (if unproven) treatment.
End of Trial and Open-Label Extension (OLE) Frequently Asked Questions ... Open Label Extension, or OLE, is a phase of a study that occurs after the randomized (blinded) portion of the trial is completed if a drug is found to have the potential for benefit. Eligible trial participants take the active form of the drug without placebo. OLE allows active drug to be given to all participants at the same time and to follow them over time.
What is an open label extension study? • NCK Pharma An open - label trial or open trial is a type of clinical trial in which both the researchers and participants know which treatment is being administered.
HUTCHMED Aug 08, 2022 · Metastatic breast, endometrial, and colorectal cancers in the U.S.: HUTCHMED initiated this open-label, multi-center, non-randomized, Phase Ib/II study in the U.S. to investigate if the addition of fruquintinib can potentially induce activity to immune checkpoint inhibitor therapy in advanced, refractory triple negative breast cancer (“TNBC ...
National Cancer Institute NCI's Dictionary of Cancer Terms provides easy-to-understand definitions for words and phrases related to cancer and medicine.
Open-label trial - Wikipedia An open-label trial, or open trial, is a type of clinical trial in which information is not withheld from trial participants. [1] In particular, both the researchers and participants know which treatment is being administered. [1]
What does open-label mean? - definitions Meaning of open-label. What does open-label mean? Information and translations of open-label in the most comprehensive dictionary definitions resource on the web. Login .
› dictionary › labelLabel Definition & Meaning - Merriam-Webster label: [verb] to affix a label to. to describe or designate with or as if with a label.








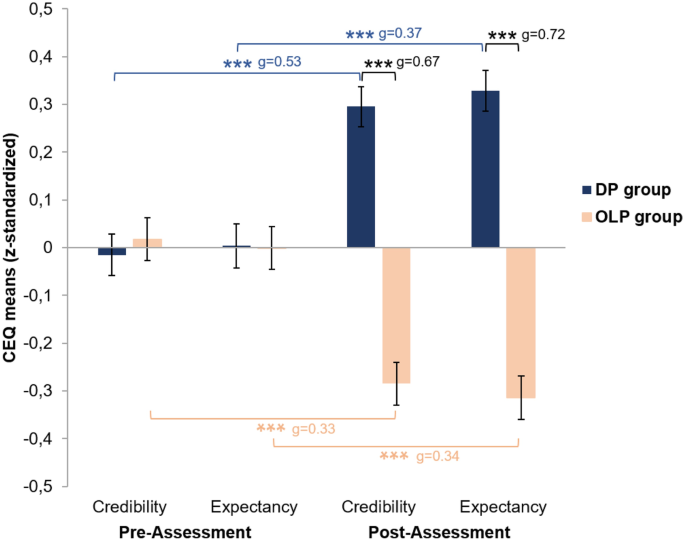


![PDF] A Novel Narrative E-Writing Intervention for Parents of ...](https://d3i71xaburhd42.cloudfront.net/f0a5f851e9565c0c95c47a95aa2678b4fb053d1c/8-Table4-1.png)








:max_bytes(150000):strip_icc()/CandlestickDefinition3-a768ecdaadc2440db427fe8207491819.png)

![PDF] Corporate governance and its contribution to risk and ...](https://d3i71xaburhd42.cloudfront.net/b41d6158254ff706ff0b4f1abe1b906bcb441399/239-Table33-1.png)






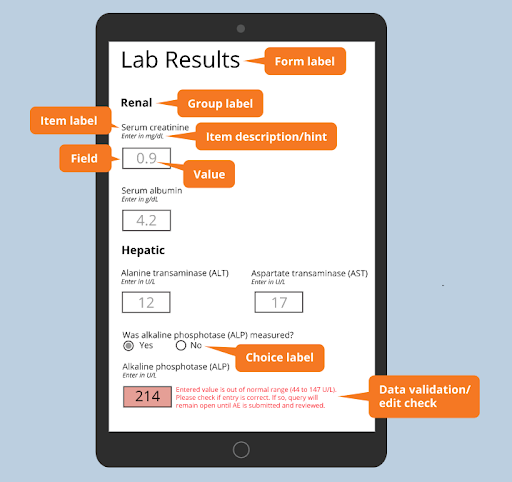



Post a Comment for "45 open-label meaning"