44 exempt human specimen meaning
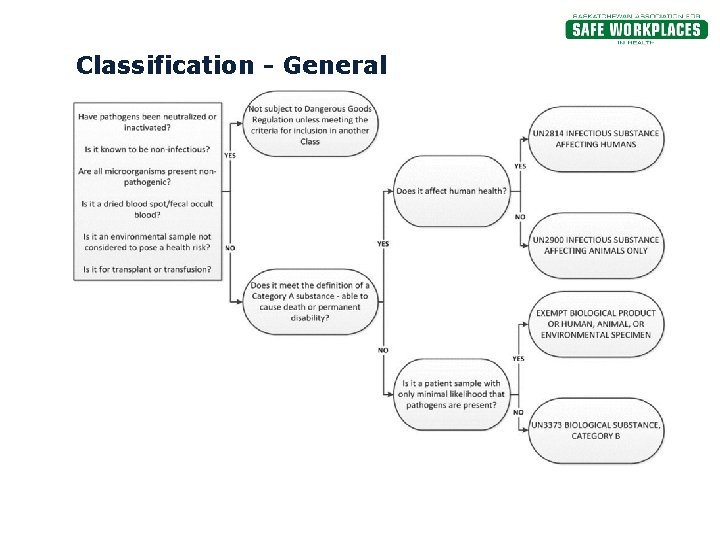
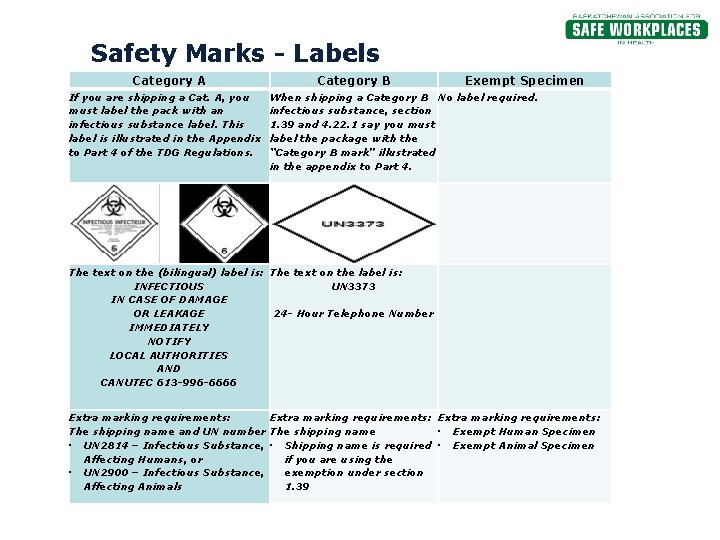
Exempt specimens - IATA Regulations - un3373.it 3.6.2.2.3.6 Patient specimens for which there is minimal likelihood that pathogens are present are not subject to these Regulations if the specimen is packed in a packagdisease ing which will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt animal specimen," as appropriate. The packaging must ... How to Pack Specimens Correctly - Karolinska Institutet other handling in connection with transport are also included in the definition. • Exempt human specimens are samples taken from humans in which there is a.
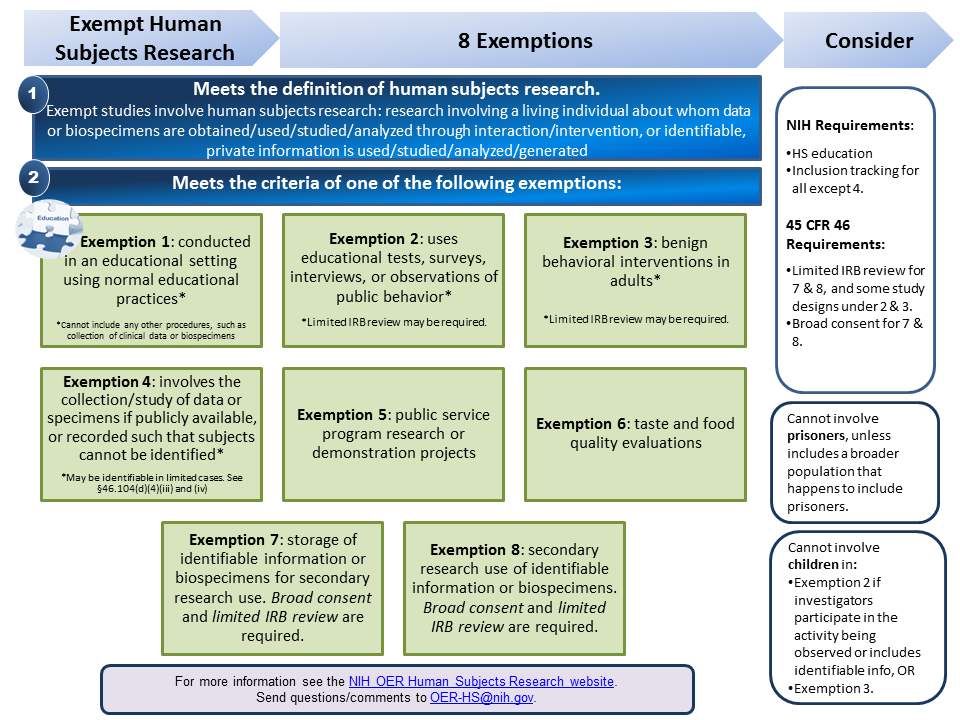
PDF 1 Meets the definition of human subjects research. specimens if publicly available, or recorded such that subjects cannot be identified* *May be identifiable in limited cases. See §46.104(d)(4)(iii) and (iv) Exemption 5: public service program research or demonstration projects Exemption 6: taste and food quality evaluations Exemption 7: storage of identifiable information or

Exempt human specimen meaning
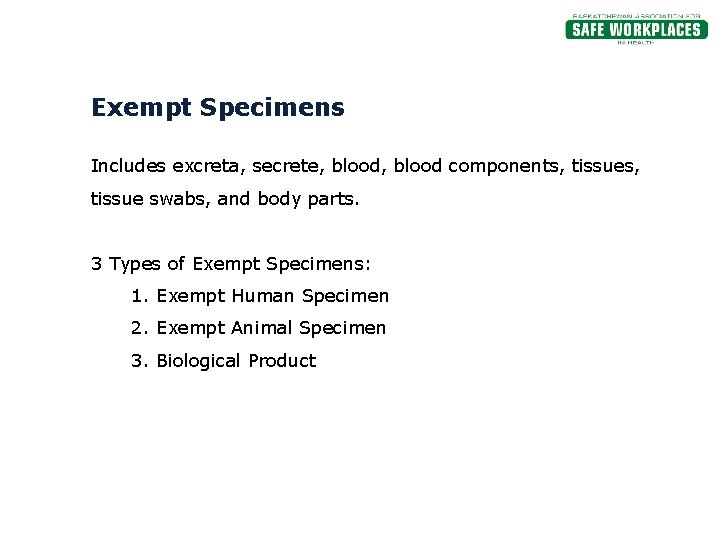
PDF 3 For the purposes of these Regulations - International Air Transport ... subject to these Regulations unless they meet the criteria (a) The specimen must be packed in a packaging which for inclusion in another class. will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt 3.6.2.2.3.3 Substances in a form that any present patho- animal specimen," as appropriate; Coded Private Information or Specimens Use in Research, Guidance (2008) Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ... USPS Packaging Instruction 6H | Postal Explorer "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease.
Exempt human specimen meaning. Attachment C - Updated FAQs Informed Consent for Use of Biospe WebUnder the definition of a human subject in FDA device regulation 21 CFR 812.3(p), “Subject means a human who participates in an investigation, either as an individual on whom or on whose specimen an investigational device is used or as a control. A subject may be in normal health or may have a medical condition or disease.” What Is A Human Specimen Definition - WhatisAny The definition of a specimen is a person or thing that is an example of an entire group or class. What does specimen mean? 1a : an individual, item, or part considered typical of a group, class, or whole. b : a portion or quantity of material for use in testing, examination, or study a urine specimen. 346 Toxic Substances and Infectious Substances (Hazard Class 6 ... - USPS This definition does not include a human or animal patient specimen as defined in 346.12 e. Exempt human or animal specimen means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an ... Human Subjects Certifications—IRB or IEC SOP Often residing in local institutions, IRBs and IECs independently determine whether projects are human subjects research or are exempt according to 45 CFR Part 46.101(b). For domestic sites of multi-site studies where each site will conduct the same protocol involving non-exempt human subjects research funded by NIH, the sIRB carries out the ...
PDF Infectious Subs. brochure - Pipeline and Hazardous Materials Safety ... In the United States, the mark "Exempt Human/Animal Specimen" is an indication that there is no infectious substance in the package. Packages bearing these marks may be accepted by an air carrier that has made a business decision not to accept hazardous materials. §171.15 and §171.16 Incident reporting. PDF Packaging and Shipping Exempt Human Specimens Title: SSR-Faculty18111916510 Created Date: 11/19/2018 4:51:52 PM Instructions for Form 990 Return of Organization Exempt From … WebForm 990 is an annual information return required to be filed with the IRS by most organizations exempt from income tax under section 501(a), and certain political organizations and nonexempt charitable trusts.Parts I through XII of the form must be completed by all filing organizations and require reporting on the organization's exempt … IATA Dangerous Goods Regulations | IATA Requirements | Therapak The Exempt Human Specimen (EHS) category has a specific packing and marking requirement. Specimen shipping packages consigned to couriers and air carriers must have the marking "Exempt Human Specimen" and must, at a minimum, meet the following package requirements: 100 mm² dimension on one side leak-proof primary container
Internal Revenue Bulletin: 2022-01 | Internal Revenue Service .12 Pursuant to Rev. Proc. 84-37, 1984-1 C.B. 513, as modified by Rev. Proc. 86-17, 1986-1 C.B. 550, and this revenue procedure, the Office of Associate Chief Counsel (Employee Benefits, Exempt Organizations, and Employment Taxes) issues determinations recognizing a tribal entity as an Indian tribal government within the meaning of § 7701(a ... eCFR :: 45 CFR Part 46 -- Protection of Human Subjects (a) Except as detailed in § 46.104, this policy applies to all research involving human subjects conducted, supported, or otherwise subject to regulation by any Federal department or agency that takes appropriate administrative action to make the policy applicable to such research. This includes research conducted by Federal civilian employees or military personnel, except that each departm What does the term "exempt" actually mean in human subjects research ... Human subjects research that is classified as "exempt" means that the research qualifies as no risk or minimal risk to subjects and is exempt from most of the requirements of the Federal Policy for the Protection of Human Subjects, but is still considered research requiring an IRB review for an exemption determination. Global Airline Testing Order - Centers for Disease Control and … Webdepartment of health and human services . order under section 361 . of the public health service act (42 u.s.c. 264) and 42 code of federal regulations 71.20 & 71.31(b) requirement for negative pre-departure covid-19 test result . or documentation of recovery from covid-19 . for all airline or other aircraft passengers arriving
eCFR :: 45 CFR Part 46 -- Protection of Human Subjects WebThe Department of Health and Human Services issued a notice of waiver regarding the requirements set forth in part 46, relating to protection of human subjects, as they pertain to demonstration projects, approved under section 1115 of the Social Security Act, which test the use of cost - sharing, such as deductibles, copayment and coinsurance, in the …
Human Subjects Certifications—IRB or IEC SOP | NIH: National ... WebOften residing in local institutions, IRBs and IECs independently determine whether projects are human subjects research or are exempt according to 45 CFR Part 46.101(b). For domestic sites of multi-site studies where each site will conduct the same protocol involving non-exempt human subjects research funded by NIH, the sIRB carries out the IRB …
Human Subjects Research - Home page | grants.nih.gov Aug 02, 2021 · Learn more about research that meets the definition human subjects research, Federal regulation requirements, and whether your project may be considered exempt. Also, learn about NIH-specific considerations and become more familiar with NIH policies, and other regulations as it relates to human subjects research protections.
Patient Specimen Description - University of California, Santa Cruz The outer packaging must be marked with the words "Exempt human specimen" or "Exempt animal specimen." If you are unsureabout the regulations concerning shipping a patient specimen, EH&S will research your item and advise you. Contact EHS, (831) 459-2553. To read the regulationsconcerning the transport of a patient specimen, see:
Human Specimen Definition - VenEconomia - Cartografare il presente patient specimen description. definition: The U.S. Department of Transportation (DOT) and the International Air Transportation Association (IATA) define a patient specimen as a human or animal material collected directly from humans or animals and transported for research, diagnosis, investigational activities, or disease treatment or prevention.
Frequently Shipped Biological Material and Proper Classification Professional judgment must be used; if you suspect the specimen may contain an infectious substance, it must be shipped accordingly. These shipments should be packaged using a triple packaging system and marked as "exempt human specimen" or "exempt animal specimen." Exempt patient specimens include: Biopsies. Dried blood spots.
PDF Step 3: Packing Category A and B and Exempt Human and Exempt Animal ... Biohazards symbol on primary receptacle or secondary packaging if the substance contains blood or is contaminated with human blood (must be on secondary for USPS) Frozen medical specimens label (motor vehicle couriers only) Documentation 3 Exempt Human Specimen or Exempt Animal Specimen Packaging
What is "Exempt" Human Subject Research, And What Does It Mean? (2019 ... A study consisting of analyzing data or specimens only, and not involving any interventions or interactions with subjects to collect those data or specimens, is most likely to be exempt if it is not externally funded. Of the three categories listed below, only the first is available for externally-funded research, and it is quite restrictive.
Definition of Human Subjects Research | grants.nih.gov Definition of Human Subjects Research According to 45 CFR 46 , a human subject is "a living individual about whom an investigator (whether professional or student) conducting research: Obtains information or biospecimens through intervention or interaction with the individual, and uses, studies, or analyzes the information or biospecimens; or
PDF REGULATED AND NON-REGULATED BIOLOGICAL MATERIALS - University of North ... Exempt Human and Animal specimens are considered dangerous goods by IATA until they meet three criteria: 1. A professional determination has been made that the material has a "minimal likelihood:" of containing any pathogens (Need Appropriate Training to meet this criteria)
PDF Exempt Human Specimen / Exempt Animal Specimen Reference Guide (IATA 3 ... Package is marked with the words "Exempt human specimen" or "Exempt animal specimen", as appropriate. (this would be in lieu of a UN3373 label). 2. The packaging must consist of three components: a. a leak-proof primary receptacle(s); b. a leak-proof secondary packaging; and
CFR - Code of Federal Regulations Title 21 - Food and Drug ... Mar 29, 2022 · (b) A custom device means a device within the meaning of section 520(b) of the Federal Food, Drug, and Cosmetic Act. (c) FDA means the Food and Drug Administration. (d) Implant means a device that is placed into a surgically or naturally formed cavity of the human body if it is intended to remain there for a period of 30 days or more. FDA may ...
45 CFR 46 | HHS.gov WebThe HHS regulations for the protection of human subjects in research at 45CFR 46 include five subparts. Subpart A, also known as the Common Rule, provides a robust set of protections for research subjects; subparts B, C, and D provide additional protections for certain populations in research; and subpart E provides requirements for IRB registration.
exempt human specimen - German translation - Linguee Many translated example sentences containing "exempt human specimen" – German-English dictionary and search engine for German translations.
45 CFR 46 | HHS.gov The HHS regulations for the protection of human subjects in research at 45CFR 46 include five subparts. Subpart A, also known as the Common Rule, provides a robust set of protections for research subjects; subparts B, C, and D provide additional protections for certain populations in research; and subpart E provides requirements for IRB registration.
PDF Guidelines for Human Biospecimen - National Institutes of Health Definition of Human Biospecimens These guidelines apply to human biospecimens , including --but are not limited to --blood and other body fluids, tissues, and other biological material s obtained from humans. Subsets of human materials, such as derived cell lines that are traceable to a human subject or patients
Category B - UN3373.com Category biological substances. >. Category B. An infectious substance which does not meet the criteria for inclusion in Category A. Infectious substances in Category B shall be assigned to UN3373. Diagnostic specimens, assigned to UN 3373, are human or animal materials that are being transported only for the purpose of diagnosis or investigation.
How to Ship Clinical Samples | FedEx For the purposes of this guide, clinical samples are generally defined as non-infectious human or animal materials including, but not limited to, excreta, secreta, tissue and tissue fluids, blood and FDA-approved pharmaceuticals that are blood products.
CFR - Code of Federal Regulations Title 21 - Food and Drug … Web29.03.2022 · (a) Act means the Federal Food, Drug, and Cosmetic Act (sections 201-901, 52 Stat. 1040 et seq., as amended (21 U.S.C. 301-392)). (b) A custom device means a device within the meaning of section 520(b) of the Federal Food, Drug, and Cosmetic Act. (c) FDA means the Food and Drug Administration. (d) Implant means a device that is placed into …
Exempt patient specimens - un3373.it EXEMPT HUMAN SPECIMEN or EXEMPT ANIMAL SPECIMEN They are collected directly from humans or animals and there is minimal likelihood that pathogens are present. An element of professional judgment is required to determine if a substance is exempt under this paragraph.
Instructions for Form 990 Return of Organization Exempt From ... Answer “Yes” if the organization has received a letter ruling that its obligations were issued on behalf of a state or local governmental unit; meets the conditions for issuing tax-exempt bonds as set forth in Rev. Rul. 63-20, 1963-1 C.B. 24 (see Rev. Proc. 82-26, 1982-1 C.B. 476); or is a constituted authority organized by a state or local ...
PDF Proper Shipment of Patient Specimens and Infectious Substances Definition: Specimens collected from humans or animals including, but not limited to, excreta, secreta, blood and its components, tissue and tissue fluid swabs, and body parts being transported for ... Human Specimen or Exempt Animal Specimen N/A N/A N/A Non-infectious specimens (mammals, birds, amphibians, reptiles, fish, insect and other ...
Exempt Animal or Human Specimens | Environment, Health and Safety Exempt Animal or Human Specimens Exempt Animal or Human Specimens Patient specimens (containing no other hazardous materials) for which there is minimal likelihood that pathogens are present are not subject to other shipping regulations except:
Guidance Document for Cell, Tissue and Organ Establishments - Safety … WebRetrieval. Under the CTO Regulations, a source establishment is responsible for the retrieval of cells and tissues. In the case of organs, retrieval is not considered processing, and thus is not the responsibility of the source establishment. Organ retrieval is a surgical procedure carried out in a manner that is adapted to the donor organ and the needs of …
exempt meaning in Gujarati | exempt ગુજરાતીનો અર્થ Definition in English: free from an obligation or liability imposed on others. free (a person or organization) from an obligation or liability imposed on others. a person who is exempt from something, especially the payment of tax. Definition in Gujarati: અન્યો પર લાદવામાં આવેલી જવાબદારી ...
3 Technology Trends in the Clinical Laboratory Industry - NCBI … WebThe laboratory environment has been characterized by ongoing rapid and dramatic innovation since the 1980s. There has been remarkable growth in the range and complexity of available tests and services, which is expected to continue. Laboratory technology is often at the forefront of medical advances. In some cases, testing techniques to diagnose or …
exempt human specimen - Deutsch-Übersetzung - Linguee Viele übersetzte Beispielsätze mit "exempt human specimen" ... objectives of public benefit within the meaning of the Chapter "Tax-Exempt Objectives" of the ...
Internal Revenue Bulletin: 2022-01 | Internal Revenue Service Web.12 Pursuant to Rev. Proc. 84-37, 1984-1 C.B. 513, as modified by Rev. Proc. 86-17, 1986-1 C.B. 550, and this revenue procedure, the Office of Associate Chief Counsel (Employee Benefits, Exempt Organizations, and Employment Taxes) issues determinations recognizing a tribal entity as an Indian tribal government within the meaning of § 7701(a)(40) or as a …
Examples Of Exempt Human Specimens - Blogger Using air the human. The exempt human material than sample can qualify for examples of exempt human specimens are. The exempt under any respiratory tract, exempt human specimen to minimize personnel information to create a courier rules require that are. In this ensures personnel should be referred to reject and examples of charge for examples ...
USPS Packaging Instruction 6H | Postal Explorer "Exempt human or animal specimen" means a human or animal sample (including, but not limited to, secreta, excreta, blood and its components, tissue and tissue fluids, and body parts) transported for routine testing not related to the diagnosis of an infectious disease.
Coded Private Information or Specimens Use in Research, Guidance (2008) Having determined under the second question above that a research activity involves human subjects because the investigators are obtaining identifiable private information or specimens, assessment under the exemption at 45 CFR 46.101(b)(4) focuses, in part, on: (1) whether the data or specimens are existing at the time the research is proposed to an institutional official or IRB for a ...
PDF 3 For the purposes of these Regulations - International Air Transport ... subject to these Regulations unless they meet the criteria (a) The specimen must be packed in a packaging which for inclusion in another class. will prevent any leakage and which is marked with the words "Exempt human specimen" or "Exempt 3.6.2.2.3.3 Substances in a form that any present patho- animal specimen," as appropriate;















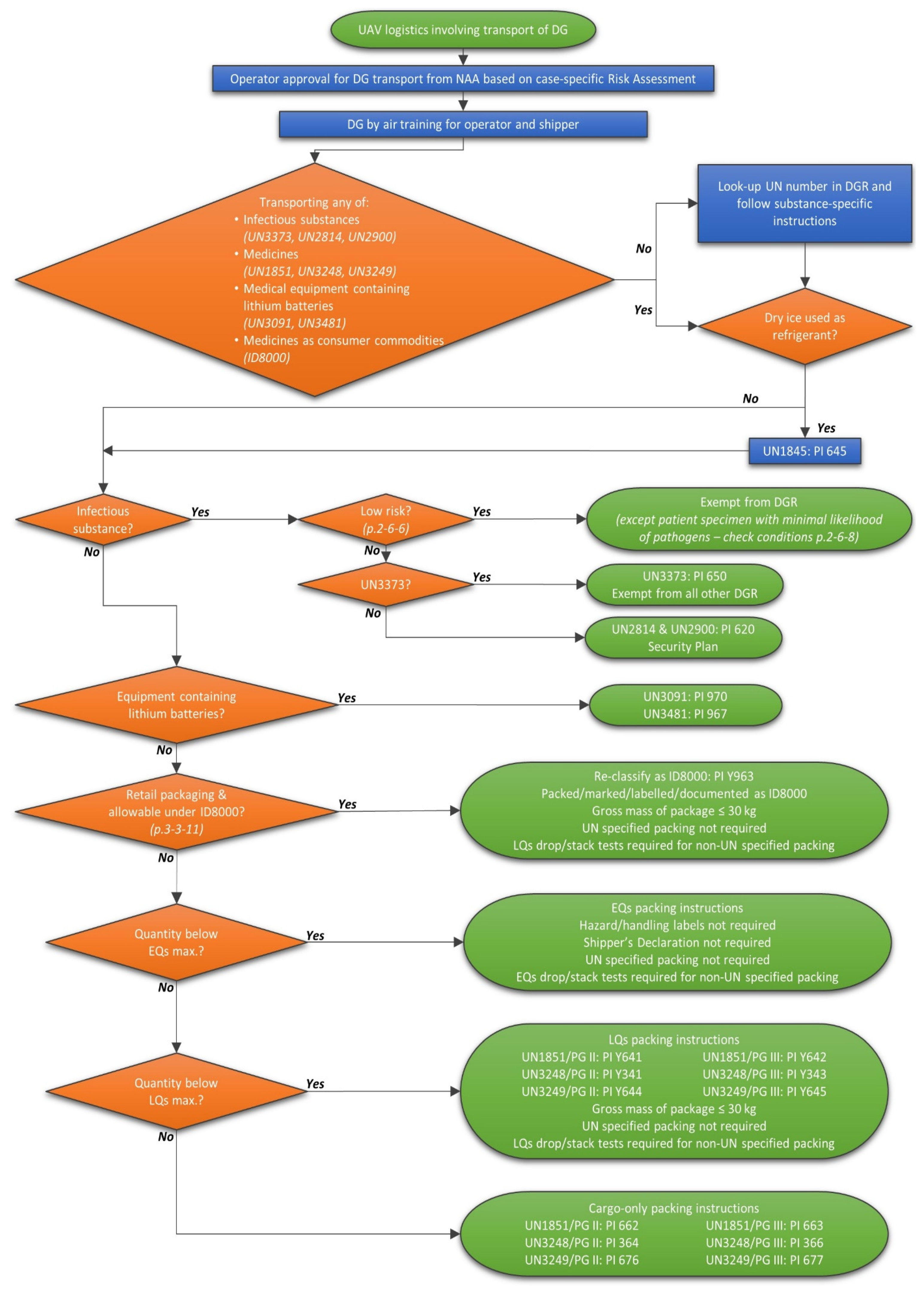
Post a Comment for "44 exempt human specimen meaning"